Discover NIS
Quantitative, High-Resolution 3D Imaging, Translatable to the Clinic
NIS is the sodium iodide symporter. It is a membrane protein that drives uptake of iodine into cells. Mammals from mouse to man express NIS endogenously in certain tissues, including the thyroid, salivary glands, gastric mucosa and mammary glands. The physiological function of NIS is to concentrate iodine in the circulation for the synthesis of thyroid hormones.
NIS is a reporter gene because NIS-expressing cells concentrate radio-labeled substrates (radiotracers) and this radioactive signal can be tracked and quantified through SPECT or PET imaging. Target cells or viruses can be engineered to express NIS in order to track them noninvasively in vivo. NIS can be used in small to large animals (and humans) for longitudinal imaging studies with non-immunogenic species-specific NIS.
Imanis offer high quality NIS tumor models and lentiviral vectors. Our tumor model cell lines can be directly implanted in vivo to facilitate tumor growth and tracking studies, whereas our lentiviral vectors can be used to generate your own NIS-expressing cells of interest for in vitro or in vivo studies.
-
Advantages of NIS
Why is NIS superior?
As a reporter gene, NIS offers several advantages, which make it an ideal choice for pre-clinical imaging that also fully translates to clinical imaging.
1. NIS imaging provides true tomographic data with unparalleled 3D spatial information and clarity on the location of the signal, which means you can see things like individual tumor nodules or infection voids.
2. Using NIS enables longitudinal studies, where the same animal can be repeatedly imaged, thus providing more meaningful data.
3. The isotopes for NIS imaging are readily available. For more information about the radiotracers used for NIS imaging see our Science Talk Blog “Choosing the best radiotracer for NIS imaging studies.”
4. NIS is a non-immunogenic self protein. Cells expressing NIS are not rejected in immunocompetent animals, permitting long-term disease modeling without immune rejection or signal loss.
5. NIS is already used in the clinic. For over 70 years, planar gamma scans of NIS activity using Iodine-123 have provided diagnosis of thyroid disorders. NIS has also been used to ablate hyperactive thyroids through targeted radiation with Iodine-131. Moreover, a variety of NIS-based therapeutics (e.g. oncolytic viruses) are currently in clinical trials.
Check out the other tabs on this page for a few real life examples and applications of NIS imagining in different biomedical fields.
-
Imaging NIS
How does NIS reporter gene imaging work?
Reagents: NIS concentrates a number of different radiotracers, several of which are commercially available and clinically approved.
- SPECT tracers: 123I (clinical),125I (pre-clinical), and [99mTc]-pertechnetate (clinical)
- PET tracers: 124I, [18F]-tetrafluoroborate, and [18F]-SO3F
For more information about radiotracers for NIS imaging, read our Science Talk Blog “Choosing the best radiotracer for NIS imaging studies.”
Equipment: SPECT or PET machines can be used to detect radiotracer signal and biodistribution of nuclear reporter-expressing cells or viruses. Combining SPECT or PET with computed tomography (CT) or magnetic resonance (MR) imaging gives the most informative results, as images can be co-registered to give anatomical localization of the radiotracer signal.
Additional considerations: Special training or certification is usually required for working with radiotracers.
NIS is endogenously expressed in the thyroid, salivary glands, mammary glands, and stomach. NIS is also not recommended for brain or CNS imaging.
Workflow:
 Theranostics’ publication on PET Imaging NIS: [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter.
Theranostics’ publication on PET Imaging NIS: [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. -
Oncology
Using NIS for Oncology Studies
With NIS imaging you can quickly and easily track the growth and metastases of cancer cells throughout the entire body and longitudinally in the same animal. Use the fully quantitative images to assess how your treatments influence tumor growth and metastases or to evaluate the efficacy of specific tumor therapies. Or combine imaging with radioiodine therapy to target and destroy cancer cells.
Tracking the growth and dissemination of tumor cells in mice
Study performed by Imanis Life Sciences
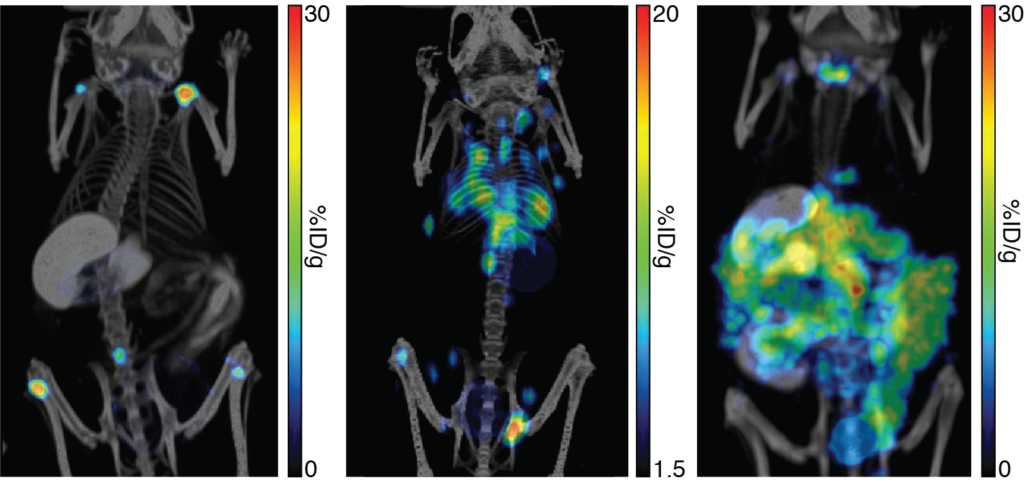 Mice were implanted with human lymphoma Nalm6-Fluc-hNIS (left), murine mammary carcinoma 4T1-mNIS (middle), or murine colorectal carcinoma CT26.WT-mNIS (right) cells. In vivo growth and dissemination of the cells was tracked by NIS imaging using 125I with SPECT/CT (left), [18F]-TFB with PET/CT (middle), or [99mTc]-pertechnetate with SPECT/CT (right).
Mice were implanted with human lymphoma Nalm6-Fluc-hNIS (left), murine mammary carcinoma 4T1-mNIS (middle), or murine colorectal carcinoma CT26.WT-mNIS (right) cells. In vivo growth and dissemination of the cells was tracked by NIS imaging using 125I with SPECT/CT (left), [18F]-TFB with PET/CT (middle), or [99mTc]-pertechnetate with SPECT/CT (right). -
Oncolytic Virotherapy
Using NIS for oncolytic virotherapy studies
Use NIS imaging to follow the biodistribution of your oncolytic virus longitudinally in the same animal or patient. Find out sooner whether your virus is targeted to the tumor and if your tumor model is permissive or resistant to your virus. Identify off-target areas of viral replication and determine whether infection got out of control by whole body imaging. NIS imaging gives you quantitative 3D representations of virus infections, so you can optimize your treatment regimen to maximize the treatment.
Longitudinal tracking of oncolytic virus therapy in a patient
Adapted from Russell et al., (2014) Mayo Clinic Proceedings. 89: 926-933. Used with permission.
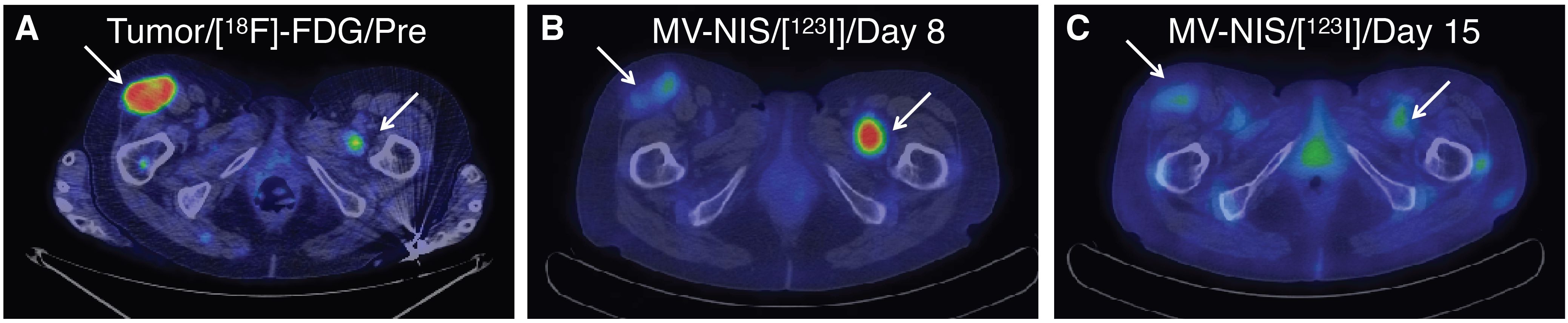
A patient with myeloma and glucose-avid plasmacytomas was treated with Measles-NIS. Pre-treatment tumor imagining was done by PET imaging with [18F]-FDG (A). Measles-NIS was tracked in vivo by SPECT/CT with 123I (B & C).
Imaging infection voids in tumors
Adapted from Miller et al., (2014) Molecular Therapy-Oncolytics. 1: 14005. Used with permission.
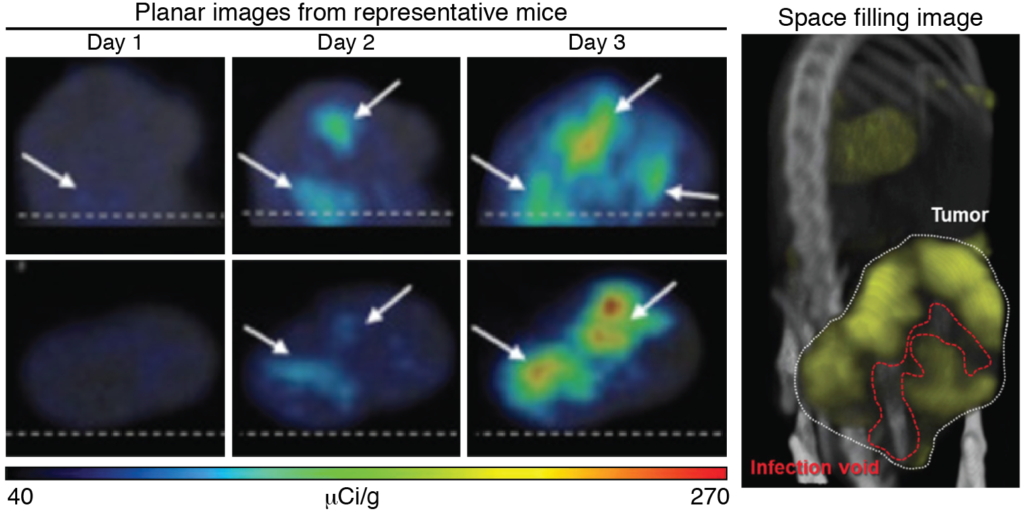
Mice with subcutaneous MPC-11 plasmacytomas were treated with a NIS-encoding virus, VSV-mIFNβ-NIS. Repeated SPECT/CT imaging using the radiotracer[99mTc]-pertechnetate tracked NIS expression, and thus virus replication, within the rumor, revealing differences in intratumoral distribution and spread of the virus. Space filling models clearly identified infection voids, which were heterogeneous between animals.
-
Regenerative Medicine
Using NIS for regenerative medicine studies
Use NIS imaging to answer important questions about the fate and efficiency of your regenerative therapies. By noninvasively tracking regenerative cells longitudinally in the same animal, you can quickly distinguish between cell death, persistence, expansion, and dispersion; learn not only where your cells are going, but also whether your therapy is working.
Longitudinal tracking of liver regeneration in a pig
Adapted from Hickey et al., (2016) Science Translational Medicine. 8: 349ra99. Used with permission.
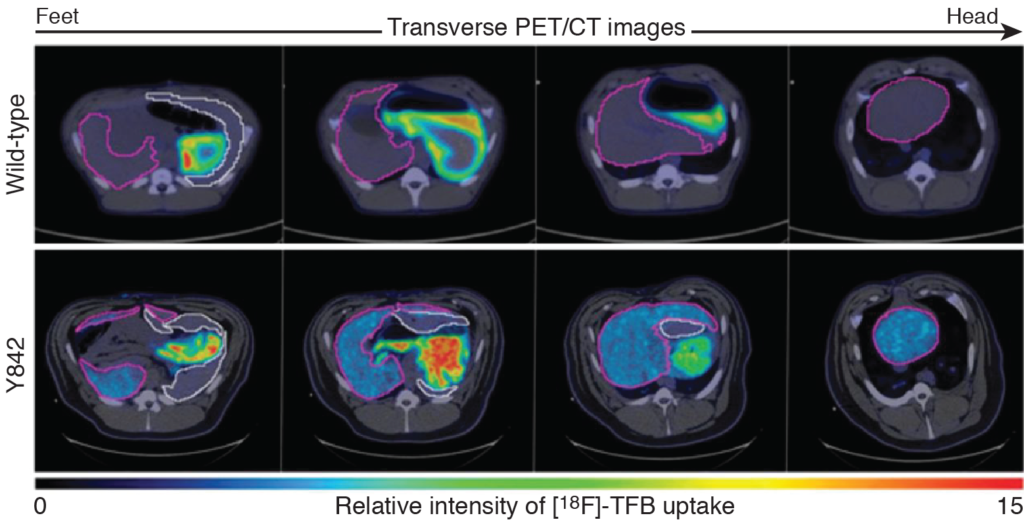
Hepatocytes were isolated from a FAV-/- pig (Y842), transduced with lentiviruses encoding FAV (to restore proper hepatocyte function) and pig NIS (to track cells), and injected into the portal vein of the pig. After 8 months, PET/CT imaging was performed using [18F]-TFB as a radiotracer. Liver regneration by the FAV/pig NIS hepatocytes was confirmed as radiotracer uptake was clearly visible in the liver (purple outlines) but not spleen (white outlines).
Tracking induced pluripotent stem cells in mice
Study performed by Imanis Life Sciences
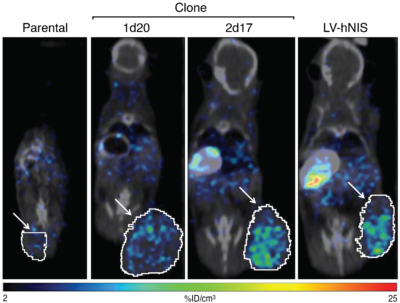
Induced pluripotent stem cells were generated with human NIS inserted either at the genomic AAVS1 safe harbor site (clones 1d20 and 2d17) or randomly in the genome (LV-hNIS). Parental or the engineered cells were implanted subcutaneously in the flanks of SCID beige mice, and after 45 days, SPECT/CT imagining was performed using [99mTc]-pertechnetate.
-
Gene Therapy
Using NIS for gene therapy studies
Use NIS imaging to quickly track vector gene expression from start to finish to determine whether your vectors are capable of efficient organ-specific gene expression. Monitor expression in the same animal over time to assess the durability of gene expression. And use the results to optimize your vector delivery methods and meet targeted expression levels.
Monitoring the global biodistribution of AAV9 vector expression in mice
Study performed by Imanis Life Sciences
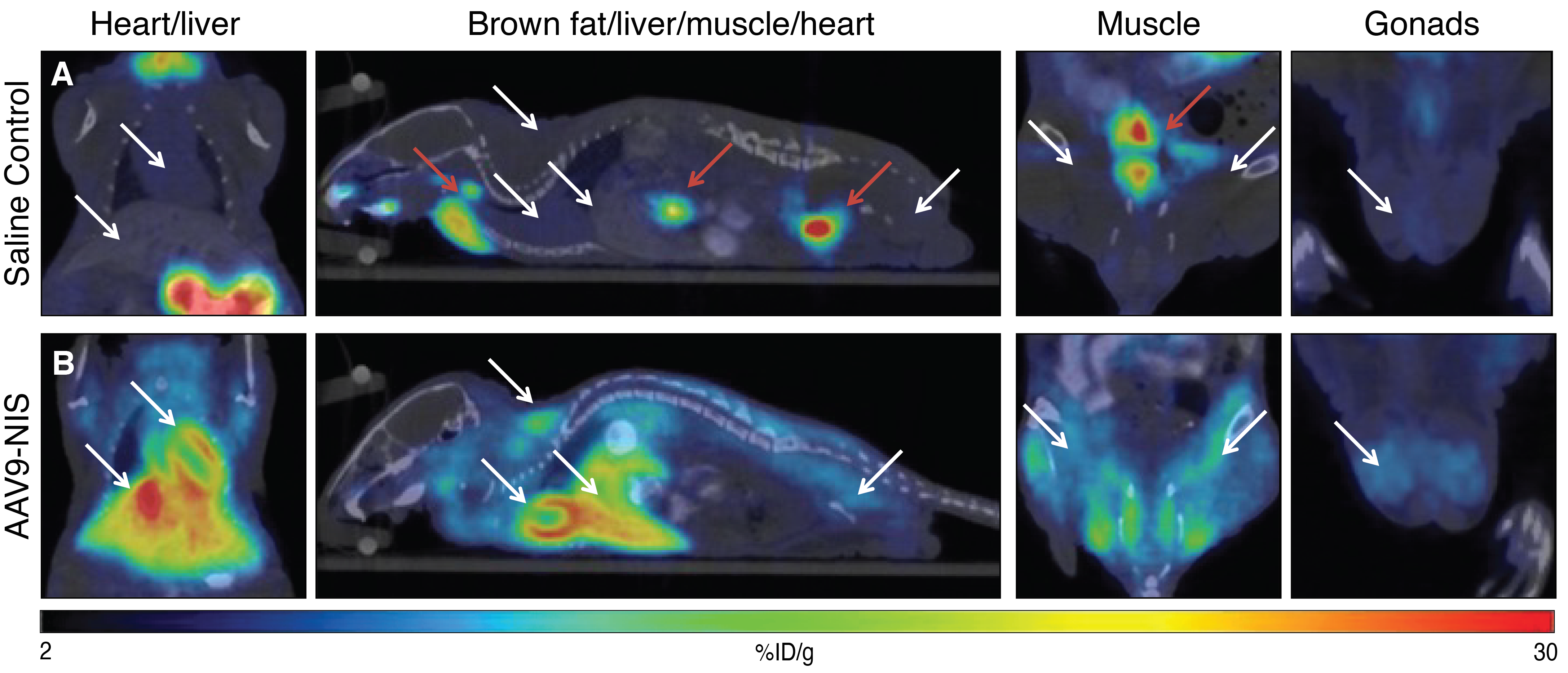
AAV9-CAG-hNIS was delivered intravenously to mice. After 24 days, PET/CT imaging was performed on control saline-treated mice (A) or AAV9-CAG-hNIS-treated mice (B) using [18F]-TFB. White arrows indicate sites of high radiotracers uptake in the AAV9-CAG-hNIS-treated mice compared to the saline control mice. Red arrows indicate areas of endogenous NIS expression and tracer excretion, including the thyroid/salivary gland, stomach, and bladder.
Optimizing AAV9 vector delivery in dog hearts
Adapted from Moulay et al., (2015) Molecular Therapy. 23: 1211-1221. Used with permission.
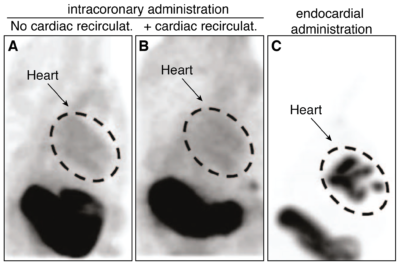
Dogs were injected with a canine NIS-expressing AAV9 vector by intracoronary administration with or without cardiac recirculation or by endocardial injection and SPECT/CT imaging was performed 2 weeks later using the radiotracer [99mTc]-pertechnetate. Imaging revealed that intracoronary administration led to little cardiac signal, but endocardial administration resulted in strong cardiac expression.

