Measles-GFP
| Strain | Edmonston vaccine lineage |
| Transgene | Green fluorescent protein (GFP) |
| Titer | >1e6 TCID50/mL (see description) |
| Risk Group | 2 |
-
Description
This is a ready to use oncolytic virus preparation of Edmonston vaccine lineage Measles virus. The virus encodes green fluorescent protein (GFP) inserted upstream of the nucleocapsid (N) gene1. The purchased virus is ready to use for generation of new viral stocks.

The virus titer is functional infectious virus, not total virus particles, in clarified supernatant. The titer is determined using an end-point dilution assay that measures the amount of virus required to produce cytopathic effects in 50% of infected cells (tissue culture infective dose per mL).
References:
1Myers et al. Hum Gene Ther. 2003 Nov, 14(16):1565-77. -
Propagation
Basic protocol
(All volumes are given for a T75 flask; increase or decrease as needed.)
1. Seed producer cells (e.g. Vero) in complete medium at an appropriate density to achieve 80-90% confluency at the time of infection and incubate in an appropriate incubator.
2. Thaw the virus stock on ice.
3. In a microcentrifuge tube, prepare virus at a MOI of 0.02 in 3 mL total serum free media.
4. Remove culture medium from cells and replace with prepared virus. Return cells to incubator.
5. After 2-3 hours, remove virus innoculum and replace with 12 mL complete medium. Return cells to incubator.
6. Harvest supernatant when 80-90% of cell monolayer has formed syncytia; usually 2-3 days post-infection.
7. Perform 2 freeze-thaw cycles. Aliquot virus supernatant and store at -80C. Determine virus titer using an appropriate method. -
Transgene Validation
Infectivity
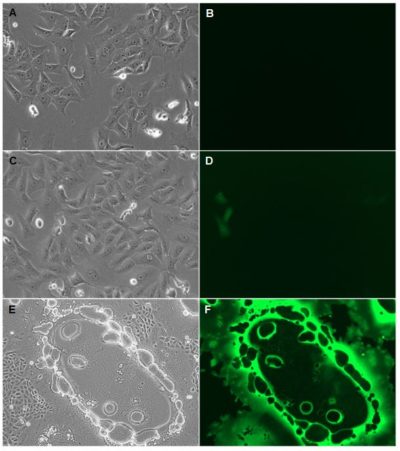 (A-B) Vero cells were mock infected
(A-B) Vero cells were mock infected(C-F) Infected with Measles-eGFP at an MOI of 0.2
(A-D) Photos were taken at 200x magnification after 24 hours
(E-F) 100x magnification after 48 hours