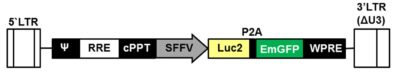
LV-SFFV-Luc2-P2A-EmGFP
| Reporter Gene | Firefly luciferase (Luc2) Emerald green fluorescent protein (EmGFP) |
| Selection Gene | None |
| Promoter | Spleen focus-forming virus (SFFV) |
| Titer | At Least 5e6 Tu/mL (see description) |
-
Description
This is a ready-to-use lentivirus preparation. The virus encodes the firefly luciferase (Luc2) cDNA linked to the emerald green fluorescent protein (EmGFP) cDNA via a P2A cleavage peptide; the spleen focus-forming virus (SFFV) promoter transcriptionally controls expression of the Luc2-P2A-EmGFP cDNA.
The virus titer is functional infectious virus, not total virus particles, and indicates the number of transducing units (TU) per mL. The titer is determined using a WPRE probe-based qPCR assay that measures the number of copies of lentiviral vector stably integrated into the genome of transduced host cells. Unlike traditional p24 ELISA titrations, which measure both functional and non-functional lentiviral vector particles, this method measures the amount of functional lentiviral vector, which is more precise and relevant.
To increase biosafety, Imanis lentiviral vectors are self-inactivating (SIN). The viral enhancer and promoter have been deleted, which causes transcription inactivation of the LTR in the provirus; mobilization of replication competent viruses is prevented, while regulated expression of the genes occurs from the internal promoters without cis-acting effects from the LTR.1
References:
1Miyoshi et al. J Virol 1998. 72:8150-8157. -
Transgene Validation
Luciferase Expression
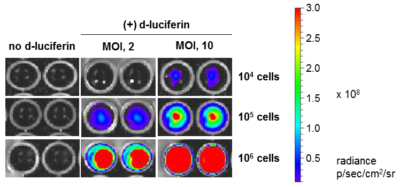
HeLaH1 cells were transduced with LV-Luc2-P2A-EmGFP at the indicated MOIs. To measure luciferase expression, 104, 105, or 106 cells were seeded in wells of a 96-well plate and 3 mg/mL of d-luciferin was added to the indicated wells. The plate was immediately imaged using the IVIS Spectrum imaging system.
GFP Expression
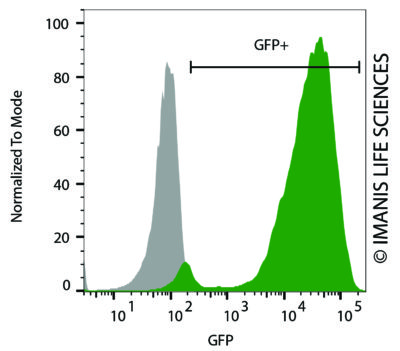 HeLaH1 cells were transduced with LV-Luc2-P2A-EmGFP (MOI = 10). The transduced cells (green) and untransduced control cells (grey) were fixed with paraformaldehyde and analyzed by flow cytometry.
HeLaH1 cells were transduced with LV-Luc2-P2A-EmGFP (MOI = 10). The transduced cells (green) and untransduced control cells (grey) were fixed with paraformaldehyde and analyzed by flow cytometry.Cell Photos
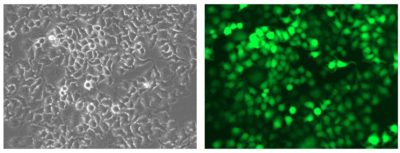
HeLaH1 cells were transduced with LV-Luc2-P2A-EmGFP (MOI = 10). Matched brightfield (left) and fluorescent (right) photos were taken at 200x magnification.
-
Transduction
Basic protocol
(Volumes are given for a 6-well plate; increase or decrease as needed.)
1. Seed cells in complete medium at an appropriate density to achieve 60-70% confluency at the time of transduction and incubate in an appropriate incubator.
2. Thaw the lentiviral vector stock on ice.
3. In a microcentrifuge tube, prepare virus at different MOIs (e.g. 1, 3, 10, and 30) in 1 mL total serum free media in the presence of Polybrene® (Imanis #REA001).
[Note: The final concentration of Polybrene® in the lentiviral vector mixture should be 4-8 µg/mL. Polybrene® can be cytotoxic to some cells and it is not advisable to incubate these cells with Polybrene® overnight; test cells in advance by incubating them with different concentrations of Polybrene®.]
4. Remove culture medium from cells and replace with prepared lentiviral vector.
5. Return cells to incubator.
6. After 3-4 hours, add 1 mL complete medium to each well and return to incubator.
[Note: if cells are sensitive to Polybrene®, the transduction mixture can be removed entirely and replaced with 2 mL of complete medium.]
7. 48-72 hours after transduction, check transgene expression according to an appropriate protocol.Spin infection
(Volumes are given for a 6-well plate; increase or decrease as needed.)
1. Seed cells in complete medium at an appropriate density to achieve 60-70% confluency at the time of transduction and incubate in an appropriate incubator.
2. Thaw the lentiviral vector stock on ice.
3. In a microcentrifuge tube, prepare virus at different MOIs (e.g. 1, 3, 10, and 30) in 1 mL total serum free media in the presence of Polybrene® (Imanis #REA001).
[Note: Polybrene® is added to the transduction mixture to enhance transduction efficiency1. The final concentration of Polybrene® in the transduction medium should be 4-8 µg/mL. Polybrene® can be cytotoxic to some cells and it is not advisable to incubate these cells with Polybrene® overnight; test cells in advance by incubating them with different concentrations of Polybrene®.]
4. Remove culture medium from cells and replace with prepared lentiviral vector.
5. Centrifuge the plate at 800 x g for 30-60 min.
6. Return cells to incubator.
7. After 3-4 hours, add 1 mL complete medium to each well and return to incubator.
[Note: if cells are sensitive to Polybrene® the transduction mixture can be removed entirely and replaced with 2 mL of complete medium.]
8. 48-72 hours after transduction, check transgene expression according to an appropriate protocol.Transduction tips
1. Avoid repeated freeze-thaw cycles of the lentiviral vector, as this causes the titer of the virus to drop and decreases transduction efficiency.
2. The optimal MOI will vary between different cell types. Typically, primary cells require higher MOIs than established cell lines.
3. Transduction efficiency can be enhanced by including Polybrene® in the lentiviral vector mixture1, using a spin infection2, and by reducing serum in the transduction mixture3.References:
1Konopka et al. J Gen Virol. 1991. 72:2685-2696.
2O’Doherty et al. J Virol. 2000. 74:10074-10080.
3Andreadis and Palsson. Human Gene Ther. 1997. 8:285-291. - Datasheet/COA