Nalm6-Fluc-hNIS
| Species | Human |
| Cell Type | Lymphoma |
| Transgene | Firefly luciferase (Fluc) Human Sodium Iodide Symporter (hNIS) |
-
Description
Parental Nalm6 cells were transduced with LV-Luc2-P2A-hNIS (Imanis #LV023) encoding the firefly luciferase (Fluc) cDNA under the spleen focus- forming virus (SFFV) promoter and linked to the human sodium iodide symporter (hNIS) cDNA via a P2A cleavage peptide. A high Fluc and hNIS expressing population was generated by two rounds of selection using a methylcellulose based semi-solid medium.
This cell line has been tested for mycoplasma contamination and is certified mycoplasma free.
The parental Nalm6 cell line was authenticated and certified free of interspecies cross contamination by STR profiling.
-
Reporter Validation
In Vivo Imaging
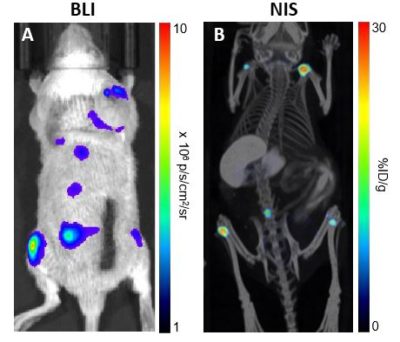 SCID beige mice were injected intraveneously with 1 x 106 Nalm6-Fluc-hNIS (CL143) cells. (A) Bioluminescent imaging was performed after 33 days. (B) NIS imaging by SPECT/CT using I-125 was performed after 25 days.
SCID beige mice were injected intraveneously with 1 x 106 Nalm6-Fluc-hNIS (CL143) cells. (A) Bioluminescent imaging was performed after 33 days. (B) NIS imaging by SPECT/CT using I-125 was performed after 25 days.Morphology
 Low and high density cell morphology (200x)
Low and high density cell morphology (200x)Luciferase Expression
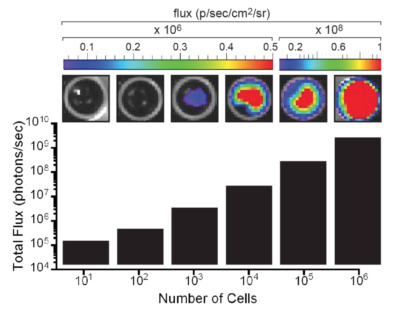
The indicated number of cells were placed in wells of a 96-well plate. After the addition of 3 mg/mL d-luciferin, the plate was immediately imaged using an IVIS Spectrum. The total flux (photons/sec) was plotted as a function of cell number.
-
Growth Conditions
Complete Growth Medium: RPMI-1640 Medium (RPMI) containing 10 mM HEPES supplemented with 10% FBS, 1% Penicillin/Streptomycin.
The cells should be grown in the indicated medium and subcultured as needed to maintain a density between 3 x 105 and 3 x 106 cells/mL. The cells can be passaged by dilution in fresh complete growth medium. Regular passaging using centrifugation is recommended to limit the amount of debris in cultures.
-
Usage Information
These cells are suitable for in vitro and in vivo experimentation.
Nalm6 cells form tumors and metastases at multiple sites, including the liver, lymph nodes, spleen, long bones, periodontal region, and bone marrow, upon implantation into immunocompromised mice1,2.
The Fluc and hNIS transgenes facilitate in vivo noninvasive bioluminescent and high resolution 3D SPECT/PET imaging, respectively, of implanted cells.
These cells were generated via lentiviral vector transduction. The lentiviral vector used for transduction was a self-inactivating (SIN) vector in which the viral enhancer and promoter have been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR2. Nevertheless, all work with these cells should be performed under biosafety-level 2 (BSL2) conditions by trained personnel. Institutional requirements may permit handling of these cells under BSL1 conditions if certain criteria are met.
-
Datasheet/COA
Lot Number CL-IM152