U266B1-Fluc-Puro
| Species | Human |
| Cell Type | Multiple Myeloma |
| Transgene | Firefly luciferase (Fluc; Luc2) |
| Selection Gene | None |
-
Description
Parental U266B1 cells were transduced with LV-SFFV-Luc2-P2A-Puro (Imanis #LV012) encoding the firefly luciferase (Fluc; Luc2) cDNA under the spleen focus-forming virus (SFFV) promoter and linked to the puromycin resistance gene (Puro) via a P2A cleavage peptide. A high Fluc expressing population was generated by selection using puromycin followed by selection with a methylcellulose based semi-solid medium.
This cell line has been tested for Mycoplasma contamination and is free of Mycoplasma species.
The parental U266B1 cell line was licensed and purchased directly from ATCC*.
*The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
-
Characterization
Morphology
 Phase cell photos taken at 200x magnification
Phase cell photos taken at 200x magnificationLuciferase Activity
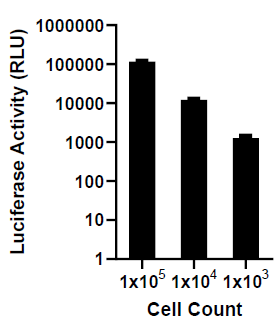 The indicated number of cells were placed in wells of a 96-well plate. After the addition of 300µg d-luciferin, bioluminescence was immediately read using a plate reader. The total bioluminescence (RLU) was plotted as a function of cell number.
The indicated number of cells were placed in wells of a 96-well plate. After the addition of 300µg d-luciferin, bioluminescence was immediately read using a plate reader. The total bioluminescence (RLU) was plotted as a function of cell number.Surface Receptor Profiling
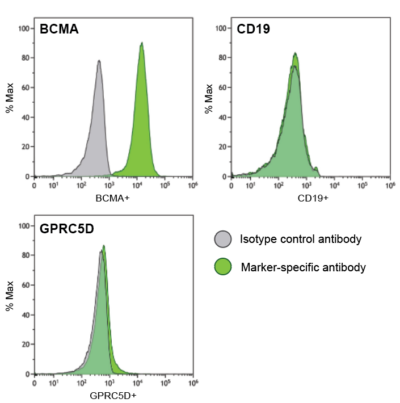 U266B1-Fluc-Puro (green) cells were stained with isotype control antibody (grey) or antibody specific to BCMA (top, left), CD19 (top, right), or GPRC5D (bottom) and analyzed by flow cytometry.
U266B1-Fluc-Puro (green) cells were stained with isotype control antibody (grey) or antibody specific to BCMA (top, left), CD19 (top, right), or GPRC5D (bottom) and analyzed by flow cytometry. -
Growth Conditions
Complete Growth Medium: ATCC-formulated RPMI-1640 supplemented with 15% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin.
These cells should be grown in the indicated medium and subcultured as needed to maintain a density between 5 x 105 and 2 x 106 cells/mL. The cells can be passaged by dilution in fresh medium, with occasional passaging using centrifugation to limit the amount of debris in cultures.
-
Usage Information
These cells are suitable for in vitro and in vivo experimentation. The Fluc transgene facilitates in vivo noninvasive bioluminescent imaging of implanted cells. EmGFP is not recommended for in-life imaging, but can be used for post-mortem confirmatory assays. Both Fluc and EmGFP facilitate quantitation of cells in vitro.
These cells were generated via lentiviral vector transduction. The lentiviral vector used for transduction was a self-inactivating (SIN) vector in which the viral enhancer and promoter have been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR1. Nevertheless, all work with these cells should be performed under biosafety-level 2 (BSL2) conditions by trained personnel. Institutional requirements may permit handling of these cells under BSL1 conditions if certain criteria are met.
1Miyoshi, H et al. (1998). Development of a self-inactivating lentivirus vector. Journal of Virology 72: 8150-8157.
-
Datasheet/COA
Lot Number IMP021