4T1-mNIS-Neo/iRFP-Puro
| Species | Mouse |
| Cell Type | Mammary Carcinoma |
| Transgene | Mouse sodium iodide symporter (mNIS) Near-infrared fluorescent protein (iRFP; ex/em: 690/713nm) |
| Selection Genes | Neomycin (Neo) and Puromycin (Puro) resistance |
-
Description
4T1-mNIS-Neo/iRFP-Puro is a polyclonal population of the mouse mammary carcinoma cell line 4T1 (ATCC® CRL-2539™). To achieve stable reporter expression in the polyclonal population, parental 4T1 cells were transduced with LV-mNIS-P2A-Neo (LV025) and LV-iRFP-P2A-Puro (LV032) and selected using G418 and puromycin. LV-mNIS-P2A-Neo (LV025) expresses the mouse sodium iodide symporter (mNIS) cDNA linked to the neomycin resistance gene (Neo) via a P2A self-cleavage peptide under the spleen focus-forming virus (SFFV) promoter. LV-iRFP-P2A-Puro expresses near-infrared fluorescent protein (iRFP) cDNA under the SFFV promoter and the puromycin resistance gene under the phosphoglycerate kinase promoter (PGK).
*The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
This cell line has been tested for mycoplasma contamination and is certified mycoplasma free.
The parental 4T1 cell line has been authenticated and certified free of interspecies cross contamination by short tandem repeat (STR) profiling with 9 STR loci.
Due to the immunogenicity of the reporter genes in this cell line, we recommend using immunocompromised mice for in vivo studies.
-
Characterization
Morphology
 Low and high density cell morphology (200x)
Low and high density cell morphology (200x)NIS Expression
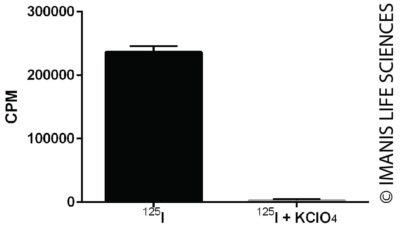 Cells were incubated with I-125 for one hour in the presence or absence of KClO4, an inhibitor of NIS mediated iodine uptake. Radioiodine concentrated within the cells was measured with a gamma counter.
Cells were incubated with I-125 for one hour in the presence or absence of KClO4, an inhibitor of NIS mediated iodine uptake. Radioiodine concentrated within the cells was measured with a gamma counter.iRFP Exprerssion
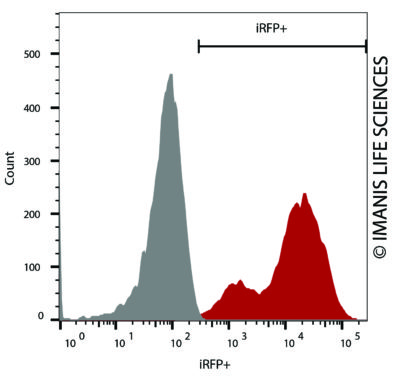 4T1-mNIS-Neo/iRFP-Puro (red) or isotype control (4T1-Fluc-Neo; grey) cells were fixed with paraformaldehyde and analyzed by flow cytometry (20,000 events).
4T1-mNIS-Neo/iRFP-Puro (red) or isotype control (4T1-Fluc-Neo; grey) cells were fixed with paraformaldehyde and analyzed by flow cytometry (20,000 events). -
Growth Conditions
Complete Growth Medium: RPMI supplemented with 10% FBS, 1X Penicillin/Streptomycin, 2 µg/mL puromycin, and 0.1 mg/mL G418.
The addition of puromycin and G418 to the complete growth medium maintains high reporter expression over continued passage of the cells. It is highly recommended, especially if the cells undergo multiple passages prior to being used for studies.
These cells should be grown in the indicated medium and passaged when they reach confluency. For routine passaging, cells are recommended to be split at a 1:10 ratio every 3-4 days.
4T1 cells frequently clump during growth. When confluent sections of cells become large (e.g. fill an entire field of view at 10X) the cells should be passaged to minimize cell death. For healthy growing 4T1 cells, this usually occurs when the cells reach 80-90% confluency overall.
-
Usage Information
These cells are suitable for in vitro and in vivo experimentation. 4T1 cells form primary tumors that depending on the route of implantation, can metastasize to the lung, liver, lymph nodes, and brain post implantation into syngenic BALB/c mice.1 The iRFP and NIS transgenes facilitate noninvasive in vivo and ex vivo fluorescence imaging and high-resolution 3D SPECT/PET imaging, respectively, of implanted cells. To reduce background autofluorescence, mice should be fed an alfalfa-free diet for at least a week prior to imaging.
The cells can be amplified in vitro and used to generate additional frozen stocks. Cryopreservation of low passage stocks is recommended. Frozen stocks should be preserved in a designated cryopreservation medium.
These cells were generated via lentiviral vector transduction. The lentiviral vector used for transduction was a self-inactivating (SIN) vector in which the viral enhancer and promoter have been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR2. Nevertheless, all work with these cells should be performed under biosafety-level 2 (BSL2) conditions by trained personnel. Institutional requirements may permit handling of these cells under BSL1 conditions if certain criteria are met.
References:
1Pulask and Ostrand-Rosenberg. Cancer Res 1998. 58:1486-1493.
2Miyoshi et al. J Virol 1998. 72:8150-8157. -
Datasheet/COA
Lot Number CL-IM106