A20-eGFP-Neo/Fluc-Puro
| Species | Mouse (Mus musculus) |
| Cell Type | Lymphoma |
| Reporter Genes | Enhanced green fluorescent protein (eGFP), Firefly luciferase (Fluc) |
| Selection Genes | Neomycin (Neo), Puromycin (Puro) |
-
DESCRIPTION
This is a cell line derived from the murine lymphoma A20 cell line (ATCC® TIB-208TM). Parental A20 cells were transduced with 1) LV-eGFP-P2A-Neo (Imanis #LV067) encoding the enhanced green fluorescent (eGFP) cDNA under the spleen focus-forming virus (SFFV) promoter and linked to the neomycin resistance gene (Neo) via a P2A cleavage peptide and 2) LV-Fluc-P2A-Puro (Imanis #LV012) encoding the firefly luciferase (Fluc) cDNA under the SFFV promoter and linked to the puromycin resistance gene (Puro) via a P2A cleavage peptide. A high Fluc and eGFP expressing population was generated by selection using puromycin and G418 followed by selection using a methylcellulose based semi-solid medium. The lentiviral vectors are self-inactivating (SIN) vectors in which the viral enhancer and promoter have been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR1.
*The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
This cell line has been tested for mycoplasma and found negative for mycoplasma contamination.
The parental A20 cell line was authenticated and certified free of interspecies cross contamination by STR profiling.
Due to the immunogenicity of the reporter genes in this cell line, we recommend using immunocompromised mice for in vivo studies.
-
Characterization
In vivo Imaging
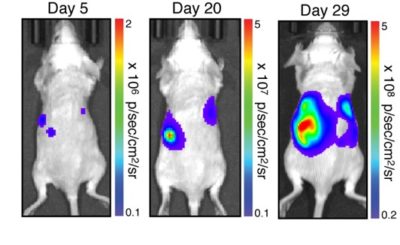
Balb/c mice were implanted with 1 x 106 A20-eGFP-Neo/Fluc-Puro cells in the tail vein. In vivo tumor growth was tracked over time using noninvasive bioluminescence imaging.
Morphology
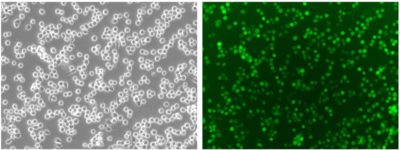 Cell photos taken at 200x magnification.
Cell photos taken at 200x magnification.Fluorescence Expression
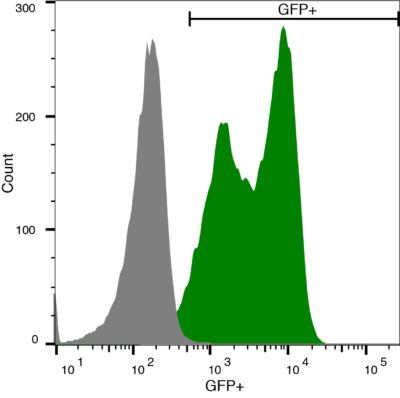
A20-eGFP-Neo/Fluc-Puro (green) or isotype control (A20 parental; grey) cells were fixed with paraformaldehyde and analyzed by flow cytometry.
Luciferase Expression
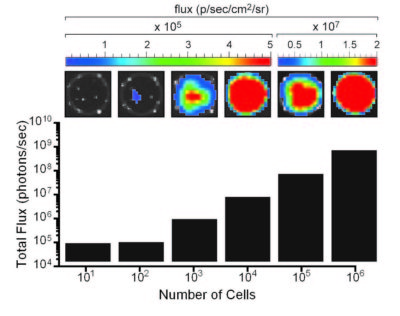
The indicated number of cells were placed in wells of a 96-well plate. After the addition of 3 mg/mL d-luciferin, the plate was immediately imaged using an IVIS Spectrum. The total flux (photons/sec) was plotted as a function of cell number.
-
GROWTH CONDITIONS
Complete Growth Medium: RPMI-1640 Medium (RPMI), 50 mM b-mercaptoethanol, 10% fetal bovine serum (FBS), 1% Penicillin/Streptomycin
To help maintain high Fluc and eGFP expression, the cells can be subcultured in the presence of 1 mg/mL G418 and 1 μg/mL puromycin. G418 and puromycin should NOT be added to the medium until a culture has been well established from the thawed cells (about 1 week). It is also recommended that a backup frozen cell stock be generated before adding G418 and puromycin to the growth medium.
-
USAGE INFORMATION
These cells are suitable for in vitro and in vivo experimentation.
A20 cells form tumors and metastases at multiple sites, including the liver, lymph nodes, spleen, bone marrow, ovaries, and peritoneal cavity, upon implantation into syngeneic Balb/c mice2.
The Fluc transgene facilitates in vivo noninvasive bioluminescent imaging of implanted cells. eGFP is not recommended for whole animal in-live imaging. Rather, samples can be collected post mortem for analysis by conventional fluorescence microscopy or flow cytometry. Fluc and eGFP are immunogenic and may cause tumor rejection in immunocompetent mice. For the most consistent results, immunocompromised mice are recommended for studies.
References:
-
Datasheet/COA
Lot Number CL-IM226