HCT116-hNIS-Neo/Fluc-Puro
| Species | Human |
| Cell Type | Colorectal Carcinoma |
| Transgenes | Human sodium iodide symporter (hNIS) Firefly luciferase (Fluc) |
| Selection Genes | Neomycin (Neo) Puromycin resistance (Puro) |
-
Description
HCT116-hNIS-Neo/Fluc-Puro is a polyclonal population of the human colorectal carcinoma cell line HCT116 (ATCC® CCL-247™). To achieve stable reporter expression in the polyclonal population, parental HCT116 cells were transduced with LV-hNIS-Neo (LV013) and LV-Fluc-P2A-Puro (LV012) and selected using G418 and puromycin. LV-hNIS-Neo encodes the human sodium iodide symporter (hNIS) cDNA linked to the neomycin resistance gene (Neo) via an internal ribosomal entry site (IRES) under the spleen focus-forming virus (SFFV) promoter. LV-Fluc-P2A-Puro encodes the firefly luciferase (Fluc) cDNA linked to the puromycin resistance gene (Puro) via a P2A cleavage peptide under the SFFV promoter.
*The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
This cell line has been tested for mycoplasma contamination and is certified mycoplasma free.
The parental HCT116 cell line has been authenticated and certified free of interspecies cross contamination by short tandem repeat (STR) profiling with 9 STR loci.
Replication Competent Lentivirus (RCL) Test (including a test report) is available for this cell line at an added cost. Contact us to learn more.
-
Characterization
Morphology
 Low and high density cell morphology (200x)
Low and high density cell morphology (200x)NIS Expression
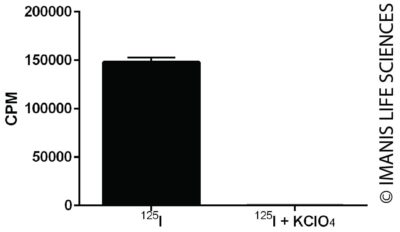 Cells were incubated with I-125 for one hour in the presence or absence of KClO4, an inhibitor of iodine uptake. Radioiodine concentrated within the cells was measured with a gamma counter.
Cells were incubated with I-125 for one hour in the presence or absence of KClO4, an inhibitor of iodine uptake. Radioiodine concentrated within the cells was measured with a gamma counter.Luciferase Expression
 104, 105, or 106 cells were placed in wells of a 96-well plate and 0.3 mg of d-luciferin was added to the indicated wells. The plate was immediately imaged using a Xenogen IVIS Spectrum.
104, 105, or 106 cells were placed in wells of a 96-well plate and 0.3 mg of d-luciferin was added to the indicated wells. The plate was immediately imaged using a Xenogen IVIS Spectrum.In Vivo Imaging
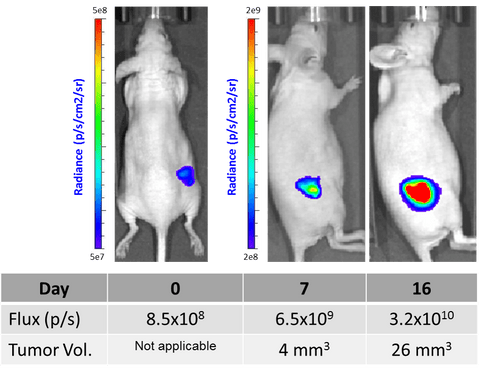 HCT116-Fluc-Puro cells (Imanis Life catalog #CL008) were injected subcutaneously into the right flanks of female NCR athymic mice. Mice were imaged at day 0 on the day of cell implantation and at days 7 and 16 using a Perkin Elmer IVIS® Spectrum system, at 10-15 minutes post intraperitoneal injection of D-luciferin at 150 mg/kg. Tumor size was measured using calipers. Data from a representative mouse (ID#4772) is shown.
HCT116-Fluc-Puro cells (Imanis Life catalog #CL008) were injected subcutaneously into the right flanks of female NCR athymic mice. Mice were imaged at day 0 on the day of cell implantation and at days 7 and 16 using a Perkin Elmer IVIS® Spectrum system, at 10-15 minutes post intraperitoneal injection of D-luciferin at 150 mg/kg. Tumor size was measured using calipers. Data from a representative mouse (ID#4772) is shown. -
Growth Conditions
Complete Growth Medium: DMEM supplemented with 10% FBS, 1X Penicillin/Streptomycin, 0.5 mg/mL G418, and 1 µg/mL puromycin.
The addition of G418 and puromycin to the complete growth medium maintains high dual reporter expression over continued passage of the cells. It is highly recommended, especially if the cells undergo multiple passages prior to being used for studies.
These cells should be grown in the indicated medium and passaged when they reach confluency. For routine passaging, cells are recommended to be split at a 1:10 ratio every 3-4 days.
HCT116 cells can detach from tissue culture surfaces upon repeated washing. Coating culture plates with poly-D-Lysine prior to use is recommended for applications involving frequent washes or culture media changes.
-
Usage Information
These cells are suitable for in vitro and in vivo experimentation. HCT116 cells are a xenograph model for colorectal carcinoma and will form primary tumors and distant metastases (lung, liver, and lymph nodes) post-implantation into immunosupressed mice.1,2 The Fluc and NIS transgenes facilitate noninvasive bioluminescent imaging and high-resolution 3D SPECT/PET imaging, respectively, of implanted cells.
The cells can be amplified in vitro and used to generate additional frozen stocks. Cryopreservation of low passage stocks is recommended. Frozen stocks should be preserved in a designated cryopreservation medium.
These cells were generated via lentiviral vector transduction. The lentiviral vector used for transduction was a self-inactivating (SIN) vector in which the viral enhancer and promoter have been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR3. Nevertheless, all work with these cells should be performed under biosafety-level 2 (BSL2) conditions by trained personnel. Institutional requirements may permit handling of these cells under BSL1 conditions if certain criteria are met.
References:
1Brattain et al. Cancer Res. 1981. 41:1751-1756.
2Yang et al. Anticancer Res. 1997. 17:3463-3468.
3Miyoshi et al. J Virol 1998. 72:8150-8157. -
Datasheet/COA
Lot Number CL-IM46