LV-mouse NIS-PGK-Puro
| Reporter Gene | Mouse sodium iodide symporter (mNIS) |
| Selection Gene | Puromycin resistance (Puro) |
| Promoters | Spleen focus-forming virus (SFFV) Phosphoglycerate kinase (PGK) promoters |
| Titer | At Least 5e6 TU/mL (see description) |
-
Description
This is a ready-to-use second generation lentiviral vector preparation. The virus encodes the mouse sodium iodide symporter (mNIS) cDNA under control of the spleen focus-forming virus (SFFV) promoter. The puromycin resistance gene is expressed by the phosphoglycerate kinase (PGK) promoter.

The lentiviral vector is a VSV.G-pseudotyped HIV-1-based lentiviral vector that is capable of transducing a broad range of cell types and species. The SFFV promoter drives high constitutive expression in numerous cell types, and works very well in animal models.
The virus titer is functional infectious virus, not total virus particles, and indicates the number of transducing units (TU) per mL. The titer is determined using a WPRE probe-based qPCR assay that measures the number of copies of lentiviral vector stably integrated into the genome of transduced host cells. Unlike traditional p24 ELISA titrations, which measure both functional and non-functional lentiviral vector particles, this method measures the amount of functional lentiviral vector, which is more precise and relevant.
To increase biosafety, Imanis lentiviral vectors are self-inactivating (SIN). The viral enhancer and promoter have been deleted, which causes transcription inactivation of the LTR in the provirus; mobilization of replication competent viruses is prevented, while regulated expression of the genes occurs from the internal promoters without cis-acting effects from the LTR.1
References:
1Miyoshi et al. J Virol 1998. 72:8150-8157. -
Transgene Validation
NIS Expression
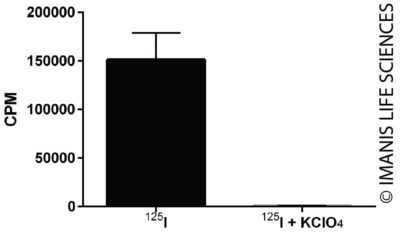 HeLaH1 cells were transduced with LV-mNIS-PGK-Puro (MOI = 10) and after three days the cells were amplified under puromycin selection. Uptake of I-125 by 2 x 105 transduced cells was assayed in the presence or absence of KClO4, an inhibitor of NIS-mediated I-125 uptake.
HeLaH1 cells were transduced with LV-mNIS-PGK-Puro (MOI = 10) and after three days the cells were amplified under puromycin selection. Uptake of I-125 by 2 x 105 transduced cells was assayed in the presence or absence of KClO4, an inhibitor of NIS-mediated I-125 uptake. -
Transduction
Basic protocol
(Volumes are given for a 6-well plate; increase or decrease as needed.)
1. Seed cells in complete medium at an appropriate density to achieve 60-70% confluency at the time of transduction and incubate in an appropriate incubator.
2. Thaw the lentiviral vector stock on ice.
3. In a microcentrifuge tube, prepare virus at different MOIs (e.g. 1, 3, 10, and 30) in 1 mL total serum free media in the presence of Polybrene® (Imanis #REA001).
[Note: The final concentration of Polybrene® in the lentiviral vector mixture should be 4-8 µg/mL. Polybrene® can be cytotoxic to some cells and it is not advisable to incubate these cells with Polybrene® overnight; test cells in advance by incubating them with different concentrations of Polybrene®.]
4. Remove culture medium from cells and replace with prepared lentiviral vector.
5. Return cells to incubator.
6. After 3-4 hours, add 1 mL complete medium to each well and return to incubator.
[Note: if cells are sensitive to Polybrene®, the transduction mixture can be removed entirely and replaced with 2 mL of complete medium.]
7. 48-72 hours after transduction, check transgene expression according to an appropriate protocol. (Note: this lentiviral vector includes a selection gene; see tips below for details.)Spin infection
(Volumes are given for a 6-well plate; increase or decrease as needed.)
1. Seed cells in complete medium at an appropriate density to achieve 60-70% confluency at the time of transduction and incubate in an appropriate incubator.
2. Thaw the lentiviral vector stock on ice.
3. In a microcentrifuge tube, prepare virus at different MOIs (e.g. 1, 3, 10, and 30) in 1 mL total serum free media in the presence of Polybrene® (Imanis #REA001).
[Note: Polybrene® is added to the transduction mixture to enhance transduction efficiency1. The final concentration of Polybrene® in the transduction medium should be 4-8 µg/mL. Polybrene® can be cytotoxic to some cells and it is not advisable to incubate these cells with Polybrene® overnight; test cells in advance by incubating them with different concentrations of Polybrene®.]
4. Remove culture medium from cells and replace with prepared lentiviral vector.
5. Centrifuge the plate at 800 x g for 30-60 min.
6. Return cells to incubator.
7. After 3-4 hours, add 1 mL complete medium to each well and return to incubator.
[Note: if cells are sensitive to Polybrene® the transduction mixture can be removed entirely and replaced with 2 mL of complete medium.]
8. 48-72 hours after transduction, check transgene expression according to an appropriate protocol. (Note: this lentiviral vector includes a selection gene; see tips below for details.)Transduction tips
1. Avoid repeated freeze-thaw cycles of the lentiviral vector, as this causes the titer of the virus to drop and decreases transduction efficiency.
2. The optimal MOI will vary between different cell types. Typically, primary cells require higher MOIs than established cell lines.
3. Transduction efficiency can be enhanced by including Polybrene® in the lentiviral vector mixture1, using a spin infection2, and by reducing serum in the transduction mixture3.
4. The presence of the puromycin resistance gene facilitates selection of transduced cells with puromycin. Selection with puromycin can be performed before or after transgene testing. The appropriate concentration of puromycin to use for selection varies with each cell line and can be determine by performing a kill curve on parental cells in parallel with transduced cells.References:
1Konopka et al. J Gen Virol. 1991. 72:2685-2696.
2O’Doherty et al. J Virol. 2000. 74:10074-10080.
3Andreadis and Palsson. Human Gene Ther. 1997. 8:285-291. -
Datasheet/COA
Lot Number LV-IM06