Nalm6-Fluc-Puro/HuCD19-Puro (low)
| Species | Human |
| Cell Type | Chronic Myelogenous Leukemia |
| Transgenes | Firefly luciferase (Fluc; Luc2), Human CD19 (HuCD19) |
| Selection Gene | Puromycin resistance (Puro) |
-
Description
The Nalm6-Fluc-Puro/HuCD19-Puro (low) cell line stably expresses firefly luciferase and levels of human CD19 that are well-below the endogenous levels normally observed in Nalm6 cells (see Characterization tab for data). The cells are designed for in vitro and in vivo studies of tumor cells expressing CD19. These cells can be used in combination with other Imanis Nalm6-Fluc-Puro cell lines that are part of the CD19 panel comprised of Nalm6-Fluc-Puro cells with various CD19 surface receptor densities:
- Nalm6-Fluc-Puro (endogenous (high) levels of CD19)
- Nalm6-Fluc-Puro/CD19-KO (negative for CD19)
- Nalm6-Fluc-Puro/HuCD19-Puro (mid) (moderate levels of CD19)
To generate this cell line, Nalm6-Fluc-Puro/CD19-KO cells were transduced with a human CD19-encoding lentivirus. A low HuCD19 expressing population was generated by selection using a methylcellulose based semi-solid medium.
This cell line has been tested for mycoplasma contamination and is certified mycoplasma free.
The parental Nalm6 cell line was licensed and purchased directly from ATCC*.
*The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
-
Characterization
Morphology
 Phase cell photo taken at 200x magnification
Phase cell photo taken at 200x magnificationLuciferase Expression
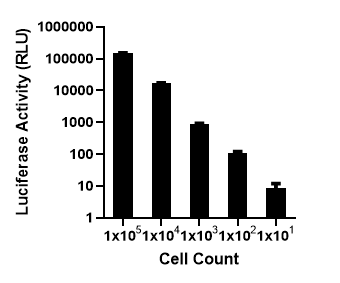
CD19 Expression
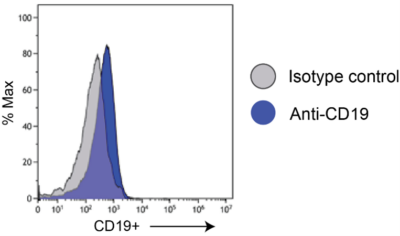
Nalm6-Fluc-Puro/HuCD19-Puro (low) cells were stained with an anti-CD19 (blue) or isotype control (grey) antibody and analyzed by flow cytometry.
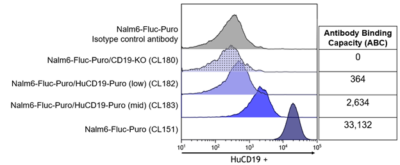 The indicated cell lines were stained with an anti-CD19 (blue) or isotype control (grey) antibody and analyzed by flow cytometry. The antibody binding capacity, representing the number of CD19 receptors on the surface of the cells was quantitated using a Quantum-Simply cellular kit.
The indicated cell lines were stained with an anti-CD19 (blue) or isotype control (grey) antibody and analyzed by flow cytometry. The antibody binding capacity, representing the number of CD19 receptors on the surface of the cells was quantitated using a Quantum-Simply cellular kit.Surface Receptor Profiling
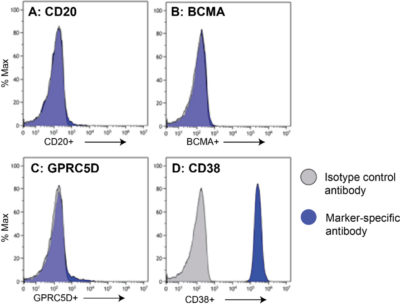 Nalm6-Fluc-Puro/HuCD19-Puro (low) cells (blue) were stained with anti-CD20 (A), anti-BCMA (B), anti-GPRC5D (C), anti-CD38 (D), or isotype control (grey; all panels) antibody and analyzed by flow cytometry.
Nalm6-Fluc-Puro/HuCD19-Puro (low) cells (blue) were stained with anti-CD20 (A), anti-BCMA (B), anti-GPRC5D (C), anti-CD38 (D), or isotype control (grey; all panels) antibody and analyzed by flow cytometry.CAR-T Activation Assay
 Jurkat T cells expressing a CD19-CAR and GFP were generated by lentivector transduction. Tranduced or untransduced parental Jurkat were incubated in a 1:1 ratio with the indicated Nalm6 cell lines and the following day flow cytometry was performed. The CD3+ Jurkat cells were analyzed for CD19CAR-GFP expression and CD69 expression, which is upregulated in activated T cells.
Jurkat T cells expressing a CD19-CAR and GFP were generated by lentivector transduction. Tranduced or untransduced parental Jurkat were incubated in a 1:1 ratio with the indicated Nalm6 cell lines and the following day flow cytometry was performed. The CD3+ Jurkat cells were analyzed for CD19CAR-GFP expression and CD69 expression, which is upregulated in activated T cells. -
Growth Conditions
Complete Growth Medium: RPMI-1640 (IMDM) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES, and 1% Penicillin/Streptomycin.
For long-term maintenance (more than two to three weeks) addition of 1 µg/mL of puromycin to the media is recommended to maintain high Fluc and HuCD19 expression.
These cells should be grown in the indicated medium and subcultured as needed to maintain a density between 5 x 105 and 3 x 106 cells/mL. The cells can be passaged by dilution in fresh medium, with occasional passaging using centrifugation to limit the amount of debris in cultures.
-
Usage Information
These cells are suitable for in vitro and in vivo experimentation. The Fluc transgene facilitates in vivo noninvasive bioluminescent imaging of implanted cells and quantitation of cells in vitro.
These cells were generated via lentiviral vector transduction. The lentiviral vectors used for transduction were self-inactivating (SIN) vectors in which the viral enhancer and promoter have been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR1. Nevertheless, all work with these cells should be performed under biosafety-level 2 (BSL2) conditions by trained personnel. Institutional requirements may permit handling of these cells under BSL1 conditions if certain criteria are met.
- Miyoshi, H et al. (1998). Development of a self-inactivating lentivirus vector. Journal of Virology 72: 8150-8157.
-
Datasheet/COA
Lot Number IMP034