VV(Li)-RlucGFP-lacZ-tRFP
| Strain | Lister (Li) |
| Transgenes | Green fluorescent protein (GFP) Renilla luciferase (Rluc) β-galactosidase (lacZ) TurboFP635-like protein (tRFP) |
| Titer | >3e6 TCID50 units/mL (see description) |
| Risk Group | 2 |
-
Description
VV(Li)-RlucGFP-lacZ-tRFP is a recombinant vaccinia virus (VV) encoding the following additional transcription units: 1) the Renilla luciferase (Rluc)-Aequorea green fluorescent protein (GFP) fusion cDNA under transcriptional control of the VV synthetic early late (pSEL) promoter, 2) the β-galactosidase (lacZ) cDNA under transcriptional control of the VV early/late (p7.5) promoter, and 3) the TurboFP635-like protein (tRFP) cDNA under transcriptional control of the VV synthetic early-late (pSEL) promoter. These additional transcription units are inserted into, and disrupt the function of, the viral F14.5L, J2R (thymidine kinase), and A56R (hemagglutinin) genes, respectively (see diagram below). The purchased virus is ready to use for generation of new viral stocks.

The virus titer is functional infectious virus, not total virus particles, in clarified supernatant. The titer is determined using an end-point dilution assay that measures the amount of virus required to produce cytopathic effects in 50% of infected cells (tissue culture infective dose per mL).
Note: A USDA permit is required for shipping Vaccinia Virus. Click here to fill out Application Form VS 16-3 to obtain a USDA APHIS VS 16-6 or 16-6A permit.
-
Propagation
This virus should be handled in a BSL2 facility by trained personnel following proper biocontainment practices.
Basic protocol
(All volumes are given for a T75 flask; increase or decrease as needed.)
1. Seed producer cells (e.g. Vero) in complete medium at an appropriate density to achieve 80-90% confluency at the time of infection and incubate in an appropriate incubator.
2. Thaw the virus stock on ice.
3. In a microcentrifuge tube, prepare virus at a MOI of 0.1 in 3 mL total serum free media.
4. Remove culture medium from cells and replace with prepared virus. Return cells to incubator.
5. After 2-3 hours, remove virus innoculum and replace with 12 mL complete medium. Return cells to incubator.
6. Harvest supernatant at 48 hours post-infection.
7. Freeze-thaw or sonicate the supernatant and cells for 3 cycles.
8. Clarify the supernatant by low-speed centrifugation and purify through a sucrose cushion.
9. Resuspend the viral pellet in 1mM TrisCl, pH 9. Determine virus titer using appropriate methods. Store at -80C. -
Transgene Validation
Infectivity
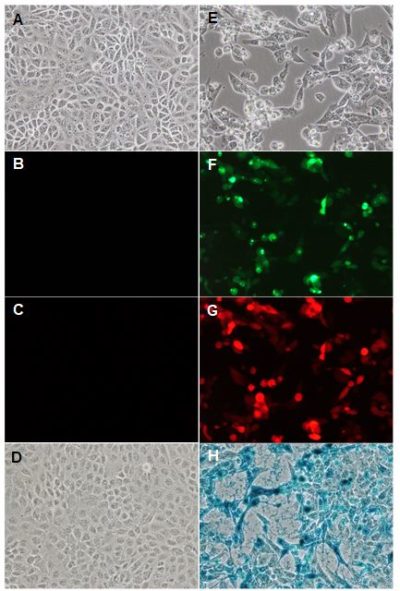 A-D: Mock-infected Vero cells.
A-D: Mock-infected Vero cells.E-H: VV(Li)-RlucGFP-lacZ-tRFP infected Vero cells (MOI 0.1) at 48 h.p.i.
D&H: β-galactosidase activity: Cells were incubated with media containing X-Gal (5-Bromo-4-Chloro-3-Indolyl β-D-Galactopyranoside).
Luciferase Expression
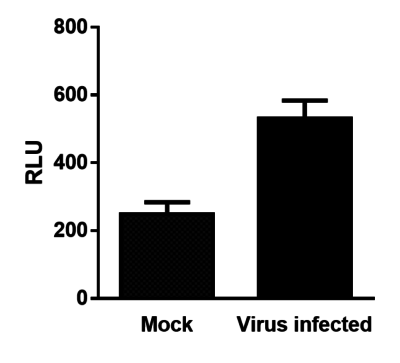 Vero cells were mock-infected or infected with VV(Li)-RlucGFP-lacZ-tRFP at an MOI of 0.1. After 48 hours, 1.25 µg of coelenterazine was added to the wells and luminescence (RLU) was immediately measured using a microplate reader.
Vero cells were mock-infected or infected with VV(Li)-RlucGFP-lacZ-tRFP at an MOI of 0.1. After 48 hours, 1.25 µg of coelenterazine was added to the wells and luminescence (RLU) was immediately measured using a microplate reader. -
Datasheet/COA
Lot Number OV-IM18