C1498-Fluc-Neo/eGFP-Puro
| Species | Mouse |
| Cell Type | Lymphoblast |
| Transgene | Firefly Luciferase (fluc) Enhanced green fluorescent protein (eGFP) |
| Selection Gene | Neomycin (neo) Puromycin (puro) |
-
Description
This is a cell line derived from the murine acute myeloid leukemia C1498 cell line (ATCC® TIB-49TM). Parental C1498 cells were transduced with 1) LV-Fluc-P2A-Neo (LV011) encoding the firefly luciferase (Fluc) cDNA under the spleen focus-forming virus (SFFV) promoter and linked to the neomycin resistance gene (Neo) via a P2A cleavage peptide, and 2) LV-eGFP-PGK-Puro (LV031) encoding the enhanced green fluorescent protein (eGFP) cDNA under the SFFV promoter and the puromycin resistance gene (Puro) under the phosphoglycerate kinase (PGK) promoter. A high Fluc- and eGFP-expressing population was generated by selection using G418 and puromycin followed by selection using a methylcellulose based semi-solid medium.
*The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
This cell line has been tested for mycoplasma contamination and is certified mycoplasma free.
The parental C1498 cell line was authenticated and certified free of interspecies cross contamination by STR profiling.
Due to the immunogenicity of the reporter genes in this cell line, we recommend using immunocompromised mice for in vivo studies.
Replication Competent Lentivirus (RCL) Test (including a test report) is available for this cell line at an added cost. Contact us to learn more.
-
Characterization
In Vivo and Post-Mortem Imaging
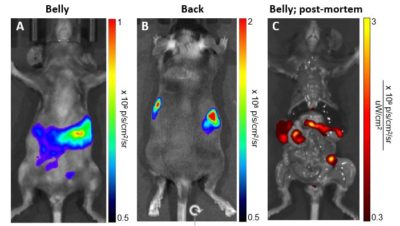
Immunocompetent syngeneic C57BL/6 mice were injected intravenously with 1 x 106 C1498-Fluc-Neo/eGFP-Puro (CL145) cells. Bioluminescent (A&B) and fluorescent (C) imaging was performed after 26 days.
Morphology
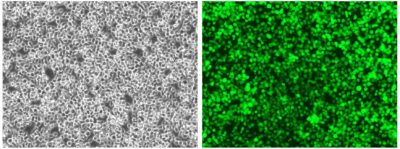 Cell photos taken at 200x magnification.
Cell photos taken at 200x magnification.Luciferase Expression
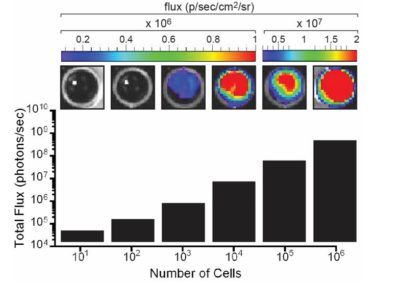
The indicated number of cells were placed in wells of a 96-well plate. After the addition of 3 mg/mL d-luciferin, the plate was immediately imaged using an IVIS Spectrum. The total flux (photons/sec) was plotted as a function of cell number.
eGFP Expression
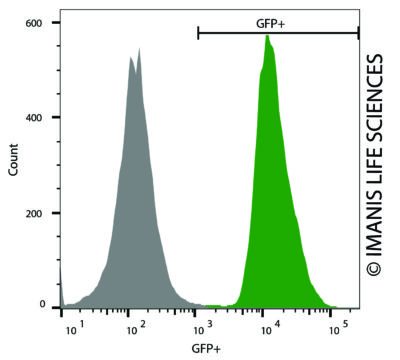 C1498-Fluc-Neo/eGFP-Puro (green) or isotype control (C1498-Fluc-Puro; grey) cells were fixed with paraformaldehyde and analyzed by flow cytometry.
C1498-Fluc-Neo/eGFP-Puro (green) or isotype control (C1498-Fluc-Puro; grey) cells were fixed with paraformaldehyde and analyzed by flow cytometry. -
Growth Conditions
Complete Growth Medium: High glucose DMEM supplemented with 10% fetal bovine serum (FBS), 1 µg/mL puromycin, 1 mg/mL G418, and 1% Penicillin/Streptomycin.
The addition of puromycin and G418 to the complete growth medium maintains high reporter expression over continued passage of the cells. It is recommended, especially if the cells undergo multiple passages prior to being used for studies.
The cells should be grown in the indicated medium and subcultured as needed to maintain a density between 3 x 105 and 2 x 106 cells/mL. The cells can be passaged by dilution in fresh medium Regular passaging using centrifugation as described above is recommended to limit the amount of debris in cultures.
-
Usage Information
These cells are suitable for in vitro and in vivo experimentation.
C1498 cells form tumors and metastases at multiple sites, including the liver, lymph nodes, spleen, bone marrow, intestines, kidneys, and central nervous system, upon implantation into syngeneic C57BL/6 mice1,2.
The Fluc transgene facilitates in vivo noninvasive bioluminescent imaging of implanted cells. eGFP is not recommended for whole animal in-live imaging. Rather, samples can be collected post mortem for analysis by conventional fluorescence microscopy or flow cytometry.
The cells can be amplified in vitro and used to generate additional frozen stocks. Cryopreservation of low passage stocks is recommended. Frozen stocks should be preserved in a designated cryopreservation medium.
These cells were generated via lentiviral vector transduction. The lentiviral vector used for transduction was a self-inactivating (SIN) vector in which the viral enhancer and promoter have been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR2. Nevertheless, all work with these cells should be performed under biosafety-level 2 (BSL2) conditions by trained personnel. Institutional requirements may permit handling of these cells under BSL1 conditions if certain criteria are met.
References:
-
Datasheet/COA
Lot Number CL-IM156